- Designed a ‘core-shell structure catalyst’ using cost-effective ruthenium to enhance commercialization potential.
- Selected as a cover paper in the prestigious catalysis journal Energy & Environmental Science.

▲ (From left) Dr. Hyun Woo Lim (First Author, SNU Department of Materials Science and Engineering), Professor Jin Young Kim (Corresponding Author, SNU Department of Materials Science and Engineering), Professor Chan Woo Lee (Corresponding Author, Kookmin University Department of Applied Chemistry), and Dr. Sung Jong Yoo (Corresponding Author, Korea Institute of Science and Technology).
Seoul National University’s College of Engineering has announced a major breakthrough in eco-friendly hydrogen production. A research team led by Professor Jin Young Kim from the Department of Materials Science and Engineering, in collaboration with Professor Chan Woo Lee from Kookmin University and Dr. Sung Jong Yoo from the Korea Institute of Science and Technology (KIST), has successfully developed an advanced electrochemical catalyst. This innovation is expected to lead the next generation of sustainable hydrogen production.
The newly developed catalyst features a ruthenium (Ru)-based nanocluster with a core-shell structure. Despite using only a minimal amount of precious metal, it delivers world-class performance and exceptional stability. Moreover, when applied to industrial-scale water electrolysis equipment, it demonstrated remarkable efficiency, highlighting its potential for commercial applications.
This groundbreaking research was published in Energy & Environmental Science (Impact Factor: 32.4, Top 0.5% in JCR), one of the most prestigious journals in the field of catalysis. Notably, the study was selected as the cover paper, further underscoring its innovation and academic significance.
Hydrogen is widely regarded as a clean energy source because it does not emit carbon dioxide when burned, making it a promising alternative to fossil fuels. One of the most efficient ways to produce eco-friendly hydrogen is through water electrolysis, which splits water into hydrogen and oxygen using electricity. Among various electrolysis methods, Anion Exchange Membrane Water Electrolysis (AEMWE) is gaining attention as a next-generation technology due to its ability to produce high-purity hydrogen. However, for AEMWE to be commercially viable, it requires catalysts that offer both high efficiency and long-term stability.
Currently, platinum (Pt) is the most widely used catalyst for hydrogen production, but its high cost and rapid degradation present significant challenges. While researchers have explored non-precious metal alternatives, these materials typically suffer from low efficiency and poor stability, making them unsuitable for industrial use.
To overcome these limitations, the research team developed a novel core-shell nanocluster catalyst based on ruthenium (Ru), which is more than twice as cost-effective as platinum. By reducing the catalyst size to below 2 nanometers (nm) and minimizing the amount of precious metal to just one-third of what is used in conventional platinum-based electrodes, the team achieved superior performance surpassing that of existing platinum catalysts.
The newly developed catalyst demonstrated 4.4 times higher performance than platinum catalysts with the same precious metal content, setting a new benchmark in hydrogen evolution reaction efficiency. Additionally, it recorded the highest performance ever reported among hydrogen evolution catalysts. Its unique foam electrode structure optimizes the supply of reaction materials, ensuring outstanding stability even under high current densities.
In industrial-scale AEMWE testing, the new catalyst required significantly less power compared to commercial platinum catalysts. This result solidifies its potential as a game-changing solution for next-generation water electrolysis technology.
The development process involved several key innovations. First, the research team treated a titanium foam substrate with hydrogen peroxide to form a thin titanium oxide layer. This was followed by doping with the transition metal molybdenum (Mo). Next, ruthenium oxide nanoparticles, measuring just 1–2 nm in size, were uniformly deposited on the modified substrate.
A precise low-temperature thermal treatment induced atomic-level diffusion, forming the core-shell structure. During the hydrogen evolution reaction, an electrochemical reduction process further enhanced the material’s properties, resulting in a ruthenium metal core encapsulated by a porous reduced titania monolayer, with metallic molybdenum atoms positioned at the interface.
Looking ahead, the core-shell nanocluster catalyst is expected to significantly improve the efficiency of hydrogen production while drastically reducing the amount of precious metal required, ultimately lowering production costs. Its combination of high performance and economic feasibility makes it a strong candidate for use in hydrogen fuel cells for vehicles, eco-friendly transportation systems, hydrogen power plants, and various industrial applications.
Beyond its practical applications, this breakthrough represents a major technological advancement that could accelerate the transition from fossil fuel-based energy systems to a hydrogen-driven economy.
Professor Jin Young Kim emphasized the impact of the research, stating, “The core-shell catalyst, despite being smaller than 2 nanometers, demonstrates remarkable performance and stability. This breakthrough will contribute significantly to the development of nano core-shell device fabrication technology and hydrogen production, bringing us closer to a carbon-neutral future.”
Meanwhile, Dr. Hyun Woo Lim, the study’s first author, has been selected for the government’s Sejong Fellowship Program and continues his research as a postdoctoral fellow in Professor Kim’s lab at Seoul National University. His current focus is on further developing and commercializing the core-shell catalyst technology.
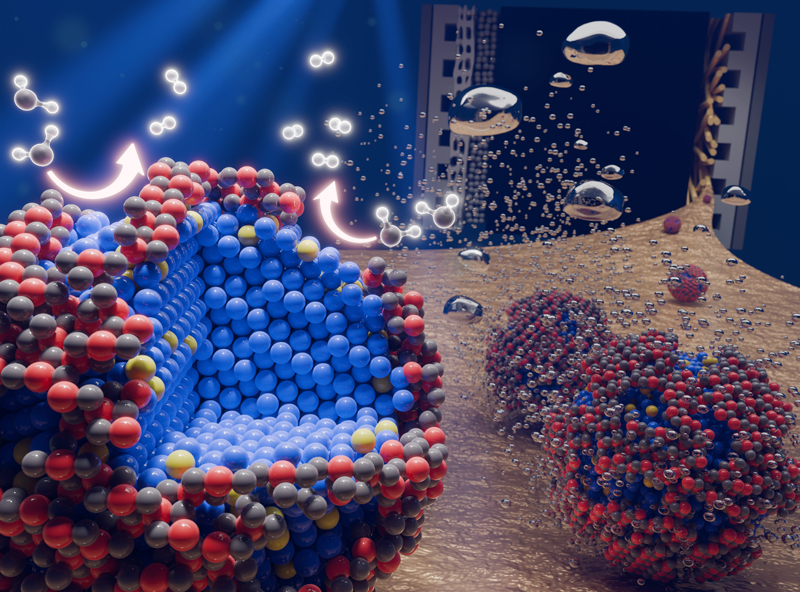
▲ Figure 1. Schematic diagram of the core-shell nanocluster
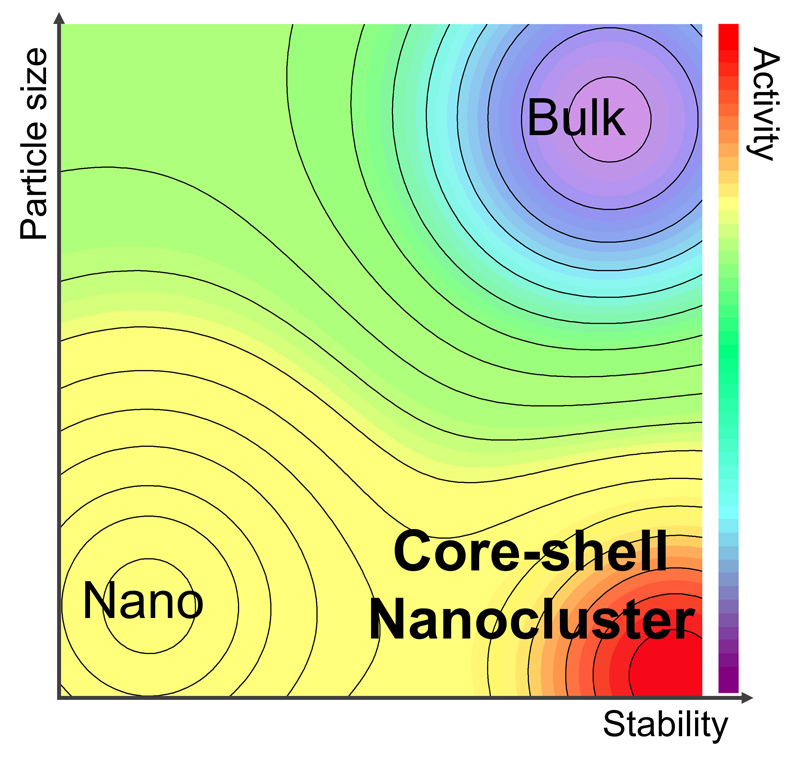
▲ Figure 2. Contour graph of the core-shell nanocluster strategy:
Nano-sized particles exhibit high activity but low stability, whereas bulk materials demonstrate high stability but low activity. By leveraging the advantages of both materials, a core-shell nanocluster material with both high activity and stability was synthesized.
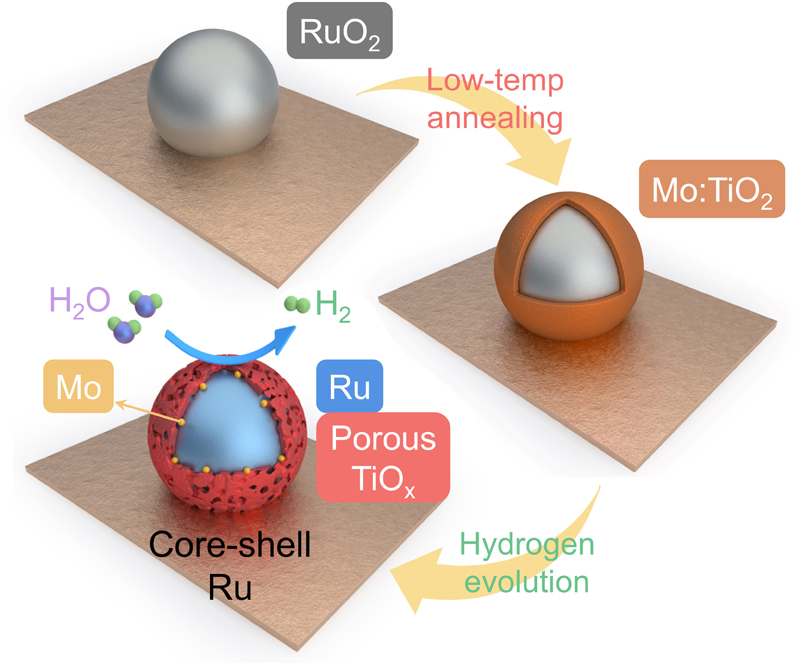
▲ Figure 3. Schematic diagram of the core-shell nanocluster formation process:
First, titanium dioxide (TiO₂) is doped with molybdenum (Mo) through initial hydrothermal synthesis. Next, additional hydrothermal synthesis is performed to deposit ruthenium oxide (RuO₂) onto the molybdenum-doped titanium dioxide substrate. A subsequent low-temperature thermal treatment (200°C) in air facilitates diffusion between titanium, molybdenum, and ruthenium oxide, forming the core-shell structure. Finally, electrochemical reduction during the hydrogen evolution reaction results in the synthesis of a unique core-shell nanocluster material.
[Reference Materials]
- Title/Journal : “A ruthenium.titania core.shell nanocluster catalyst for efficient and durable alkaline hydrogen evolution”, Energy & Environmental Science, 18, 2243-2253 (2025).
- DOI : https://doi.org/10.1039/d4ee04867a
[Contact Information]
Dr. Hyun Woo Lim, Research Institute of Advanced Materials, Seoul National University / +82-2-880-8024 / wisefriend@snu.ac.kr

